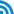Evaluation and Analysis of the Policy on Centralized Purchase of Drugs by Medical Institutions in China*
2009-10-26
Gong Seng
Based on the objectives of the Centralized Purchase of Drugs Policy, this report made an evaluation and an analysis on the impacts of the Centralized Purchase of Drugs Policy by examining the responses of diversified stakeholders. The existing problems and their causes were explored and conclusions were reached by research team independently.
In accordance with the initial objectives, the Centralized Purchased of Drug Policy, while having made some achievements, also faces some newly emerged problems and challenges, and the direct reasons for these problems and challenges result from the inherent imperfection of policy design and unsatisfactory implementation. Based on the responses of stakeholders, and compared with the objectives mentioned above, it is concluded that the Centralized Purchase of Drugs Policy has made seven major achievements, with six unresolved problems and six challenges that have newly emerged. These are summarized in Table 1.
Table 1 Objectives, Achievements and Problems of the Centralized Purchase of Drugs Policy
|
Policy Objectives |
Achievements |
Problems |
|
|
Unresolved |
Newly Emerged |
||
|
Objective 1: to standardize the purchase of drugs by medical institutions
|
1. The purchase of drugs by medical institutions is initially standardized |
1. Under-table payments to medical service providers have not disappeared 2. Delayed payments to the drug suppliers far from being resolved |
|
|
Objective 2: to regulate the circulation of drugs
|
2. The adoption of information system is enhanced in the pharmaceutical industry. 3. The channels for drug circulation have been clearly-defined and this could help contain, monitor and punish those fly-by-night companies |
3. There are still quality problems with the drugs won the bid and it is not significant for the current practice to play an active role in selecting the superior and eliminating the inferior |
1. Some low-price ordinary medicines are not available in the market
|
|
Objective 3: to control the drug prices
|
4. The market prices have been reduced and this has helped the pricing authorities to find out reasonable prices |
4. The prices of drug won the bid are still quite high
|
2. The procurement practices in many localities have been changed into price cutting 3. Setting the price ceiling has caused dissents among pricing authorities and manufacturers 4. The price gap between brand drugs and generic drugs tends to be widening |
|
Objective 4: to rectify the malpractices in drug procurement and sale
|
5. Malpractices in drug procurement and sale have been preliminarily corrected and an information system has been established for monitoring and enforcement of regulations
|
|
|
|
Objective 5: to alleviate the financial burden of drugs for patients
|
6. It has helped to contain the increasing tendency of drug costs
|
5. Patients have benefited little
|
5. The enterprises have benefited little, but bear almost all the transaction costs
|
|
Object 6: to protect legal rights and interests of stakeholders |
7. The vast majority of participants has little benefit loss
|
6. The legal rights and interests of hospitals and enterprises are not sufficient protected
|
6. The drug enterprises afford the most costs of the Centralized Purchase of Drugs while they benefit little from it. |
I. Achievements
1. The purchase of drugs by medical institutions is initially standardized
To standardize the purchase of drugs by medical institutions was first put forward as the objective of the Centralized Purchase of Drugs Policy. Before 2001, thousands of pharmaceutical manufacturers and sellers had to pay under-table payments for their drugs to more than ten thousand medical institutions. In the process of contracting, drug providers needed to negotiate one by one with medical institutions and the latter had predominant bargaining positions.
After the implementation of the Centralized Purchase of Drugs Policy, all provinces and cities under survey promulgated a series of regulations and policies to standardize the drug purchase. Although some pharmaceutical enterprises complain that many regulations are not strictly observed in practice and they need to pay under-table money when selling drugs to medical institutions, medical institutions hold that after the government agencies organize Centralized Purchase of Drugs, they no longer need to negotiate prices with drug providers. Beside, the category and quantity of drug purchased are decided by the Drug Affair Committee. This kind of collective decision-making may prevent leaders of hospital pharmacy from misdeeds. Furthermore, representatives from hospitals believe that the Centralized Purchase of Drugs reduces the public denouncement related to high drug prices toward medical institutions and doctors because hospitals are now purchasing drugs at the prices that win the bid (or the prices that are published via internet).
2 The use of information system in the pharmaceutical industry has been facilitated
In China the pharmaceutical industry is composed of drug manufacturers, drug sellers, medical institutions, pharmacies and administration authorities and so on. The value of drugs that are purchased by medical institutions amounts to three quarters of the total value of drugs in circulation. Information technology was initially adopted by tender agents with the purpose of carrying out the tender effectively and accurately. One technique is utilized in coding complicated names of drugs. At present, in some localities related governmental departments are trying to monitor all transactions through internet information system.
As a byproduct of online transaction of drugs, information system has been rapidly established and enforced in medical institutions, drug manufacturers and sellers.which help pharmaceutical enterprises to analyze the change of marketplace in real time.
3. The channels for drug circulation have been clearly-defined and this could help contain, monitor and punish those fly-by-night companies
This positive effect is cited by government officers and most enterprises from all places. In detail, the Centralized Purchase of Drugs Policy can contribute in these aspects:
(1) The channels for drug circulation have been clearly-defined
The provinces under survey have mostly published provisions about the channels for drug purchase in order to eliminate the opacity of drug circulation. In Guangdong Province, all drugs that win the bid should go through the "two invoices regulation" practice,i.e. one invoice is issued when drug manufacturers provide drugs to drug distributors and another invoice is issued when drug distributors sell drugs to medical institutions.
(2) The Centralized Purchase of Drugs Policy help to contain "drug brokers" and those fly-by-night companies
"Drug brokers" usually buy drugs from manufacturers at bottom prices and then dispense those drugs to medical institutions at high prices. However, rational drug prices are identified through centralized purchase. Consequently, the profitability of "drug brokers" is damaged. Again, fly-by-night companies have one common feature that they have no ability to distribute drugs to medical institutions. Those companies can be easily identified through the monitoring system of drug transaction by inspecting authorities.
(3) The Policy increases the concentration of market in some localities
For example, there were 500 to 600 drug dispensing companies before the implementation of the Centralized Purchase of Drugs Policy. After the Policy came into being, the top ten dispensing companies occupy about 80% of all the drugs. Another case in point is Shanghai. The share of state-holding companies is enlarged. In 2001, the state-holding companies distributed 10% of all drugs and the gross profit rate was 9-10%. In 2007, state-holding companies distributed 20% of drugs and the gross profit rate was brought down to 5-6%. Especially the low-profit, low-value drugs are increasingly more distributed by large-scale distributors. But in some provinces under survey, the concentration of market is not explicit.
…
If you need the full text, please leave a message on the website.