China's recombinant COVID-19 vaccine capable of covering virus mutations

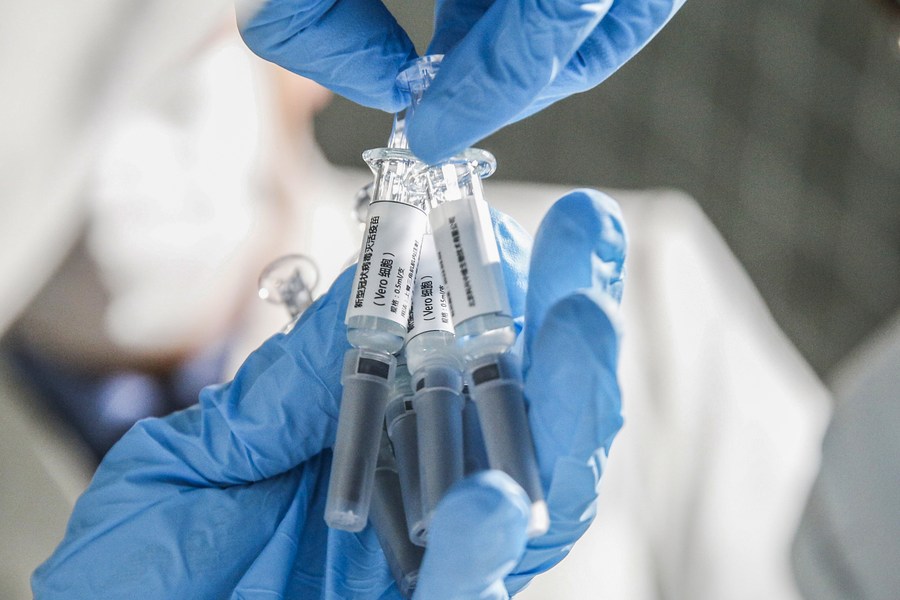
BEIJING -- China can currently realize an annual production of 300 million doses of a recombinant COVID-19 vaccine and will further expand its capacity, according to Chen Wei, head of the vaccine research team.
A genetically engineered vaccine, the recombinant vaccine developed by the Institute of Military Medicine under the Academy of Military Sciences uses a modified defective adenovirus as the vector.
The current data show a very low mutation probability of the gene that the team selected from the virus to make the vaccine.
So far, the recombinant vaccine can completely cover all the mutations of the novel coronavirus, said Chen, a researcher at the institute.
Even if the chosen gene mutates, weakening the vaccine's protective effect, the current vaccine can still be used to achieve basic immunity, and China can quickly develop a vaccine specifically targeting the mutation to enhance the immunity effect, said Chen, also an academician with the Chinese Academy of Engineering.
"It's like upgrade and patch for software," Chen said.
On March 16, the vaccine developed by Chen's team started phase-1 clinical trials, the first in the world. According to the data published in the medical journal The Lancet in May, all 108 vaccinated participants produced antibodies.
Richard Horton, editor-in-chief of The Lancet, reviewed the research results as an important milestone. He said on his Twitter page that the vaccine was safe, well-tolerated and induced a rapid immune response.
"By releasing our testing methods and indicators to the world, we have helped the researchers from other countries take fewer detours and promote global vaccine research," Chen said.
In July, the data of the vaccine's phase-2 clinical trials were also released to the world. The results of the two phases of clinical trials verified the efficacy and safety of the vaccine.
China in June approved a trial scheme for the emergency use of COVID-19 vaccines, and the recombinant vaccine was granted a green light to be given to specific people with high risk of exposure to the virus.
In August, the vaccine was granted a patent, the country's first patent for a COVID-19 vaccine.
At present, the phase-3 clinical trials are being carried out abroad to further evaluate the efficacy and safety of the vaccine among a greater number of participants, Chen said.
Although there is currently insufficient data to confirm the duration of the vaccine's efficacy, the data show that the dose vaccinated in March is still effective, Chen said, adding that related research is underway.
After phase-3 clinical trials are completed, the team will expand the production capacity to facilitate mass vaccination at any time, she said.
According to the World Health Organization, more than half of the COVID-19 vaccines that have entered phase-3 clinical trials were developed by China.
This indicates that China has been playing a vanguard role in the global research and development of COVID-19 vaccines, she said.
- Hainan's 1st batch of daily consumer goods duty-free shops for island residents opens
- Cross-Strait affairs should be settled 'like family', official says
- Whale injured in collision with fishing boat near Weizhou Island
- Lizard found on high-speed train brought by passenger, rail authorities say
- Zeiss top exec: Learn from China speed
- Ancient tomb cluster in Changsha offers a glimpse into millennia of history



































